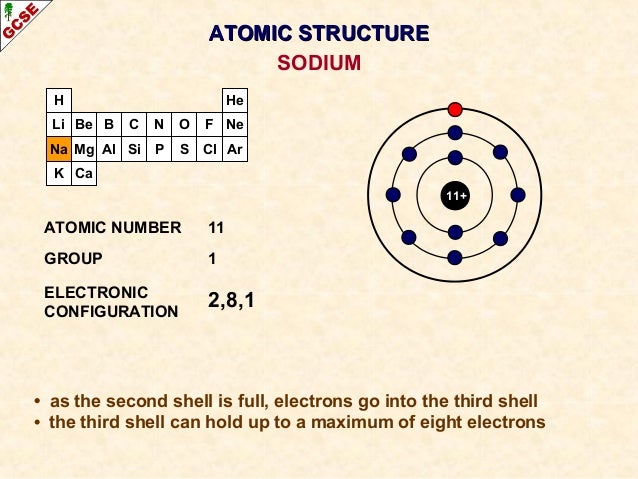
Atomic Number of Sodium is 11.
- What Is The Atomic No Of Sodium
- Atomic No Of Sodium Acetate
- Atomic No Of Sodium
- Atomic No Of Sodium Bromide
Chemical symbol for Sodium is Na. Number of protons in Sodium is 11. Atomic weight of Sodium is 22.98976928 u or g/mol. High sierra disk maker. Melting point of Sodium is 97,8 °C and its the boiling point is 892 °C.
» Boiling Point» Melting Point» Abundant» State at STP» Discovery Year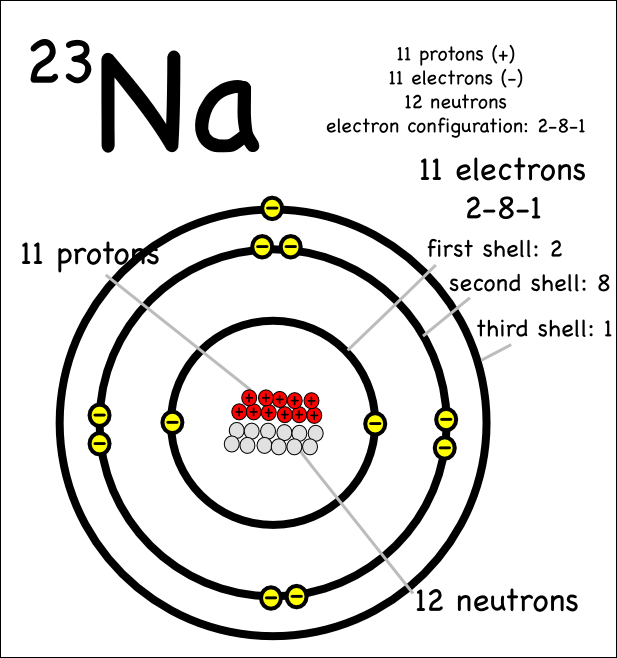
Atomic Number – Protons, Electrons and Neutrons in Sodium. Sodium is a chemical element with atomic number 11 which means there are 11 protons in its nucleus.Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. Sodium is a chemical element with the symbol Na (from Latin 'natrium') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23 Na. The free metal does not occur in nature, and must be prepared from compounds. Standard atomic weight is used to give the value of the mean of the atomic masses in a mixture of isotopes in a given sample of an element. Atomic Mass of First 30 Elements. Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic mass.
About Sodium
Sodium is the sixth most common element on Earth, and makes up 2.6% of the Earth’s crust. The most common compound is sodium chloride. This very soluble salt has been leached into the oceans over the lifetime of the planet, but many salt beds or ‘lakes’ are found where ancient seas have evaporated. Sodium has been identified in both the atomic and ionic forms in the spectra of stars, including the Sun, and the interstellar medium. Analysis of meteorites indicates that the silicate material present has an average content of approximately 4.6 atoms of sodium for every 100 atoms of silicon.
Sodium is one of the oldest known chemical elements since it is essential for human life. It is consumed by all of us in the form of salt we use for cooking many types of foods, but is a bit dangerous for human body in high amounts (causes high blood pressure). Sodium is a soft metal and is the 6th most common chemical element on our planet. Sodium is a reactive element, especially with water and other chemical compounds, that is why it can not be found in nature as metal. El capitan download bootable usb. The number of common sodium compounds is quite large (with sodium chloride being the most common of those), and they are used in plenty of industries, including toothpaste production, producing soaps, foodstuffs, as well as industrial and nuclear chemistry, and many others.
Properties of Sodium Element
| Atomic Number (Z) | 11 |
|---|---|
| Atomic Symbol | Na |
| Group | 1 |
| Period | 3 |
| Atomic Weight | 22.98976928 u |
| Density | 0.971 g/cm3 |
| Melting Point (K) | 370.87 K |
| Melting Point (℃) | 97,8 °C |
| Boiling Point (K) | 1156 K |
| Boiling Point (℃) | 892 °C |
| Heat Capacity | 1.228 J/g · K |
| Abundance | 23600 mg/kg |
| State at STP | Solid |
| Occurrence | Primordial |
| Description | Alkali metal |
| Electronegativity (Pauling) χ | 0.93 |
| Ionization Energy (eV) | 5.13908 |
| Atomic Radius | 180pm |
| Covalent Radius | 154pm |
| Van der Waals Radius | 227 |
| Valence Electrons | 1 |
| Year of Discovery | 1807 |
| Discoverer | Davy |
What is the Boiling Point of Sodium?
Sodium boiling point is 892 °C. Boiling point of Sodium in Kelvin is 1156 K.

What is the Melting Point of Sodium?
Sodium melting point is 97,8 °C. Melting point of Sodium in Kelvin is 370.87 K.
How Abundant is Sodium?
What Is The Atomic No Of Sodium
Abundant value of Sodium is 23600 mg/kg.
What is the State of Sodium at Standard Temperature and Pressure (STP)?

State of Sodium is Solid at standard temperature and pressure at 0℃ and one atmosphere pressure.
Atomic No Of Sodium Acetate
When was Sodium Discovered?
Atomic No Of Sodium
Sodium was discovered in 1807.
Atomic No Of Sodium Bromide
