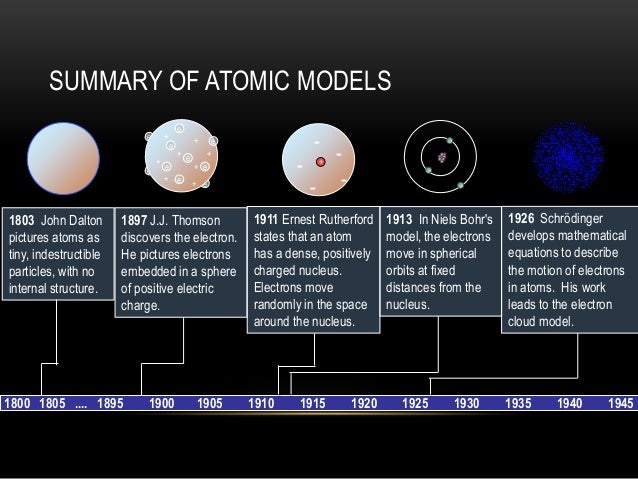
Thomson atom model
Teori Atom Thomson Model teori atom Thomson muncul setelah dikemukakannya sebuah teori Dalton pada tahun 1903. Teori atom Thomson merupakan salah satu teori yang menjelaskan bentuk atom seperti dengan bentuk roti kismis. Thomson baru-baru itu tertarik pada struktur atom yang direfleksikan dalam bukunya, yang berjudul Treatise on the Motion of Vortex Rings yang membuatnya memenangkan Adams Prize tahun 1884. Numbers for high sierra download. Bukunya yang berjudul Application of Dynamics to Physics and Chemistry terbit tahun 1886, dan pada tahun 1892 dia menerbitkan buku berjudul Notes on Recent Researches in Electricity and Magnetism.
Thomson’s model was known as the 'Plum Pudding Model” (or 'Raisin Bread Model.' ) As each atom was a sphere filled with a positively charged fluid, known as the “pudding”. Scattered in this fluid were negatively charged electrons, these were the “plums” in the pudding. Thomson's atomic atomic model was called the Plum Pudding Atomic Model, and it was based on the idea that electrons are negatively charged particles scattered through out the positively charged atom. While Thomson was right about the existence of electrons, he was wrong on where they are located within the atom. On April 30, 1897, British physicist J.J. Thomson announced his discovery that atoms were made up of smaller components. This finding revolutionized the way scientists thought about the atom.
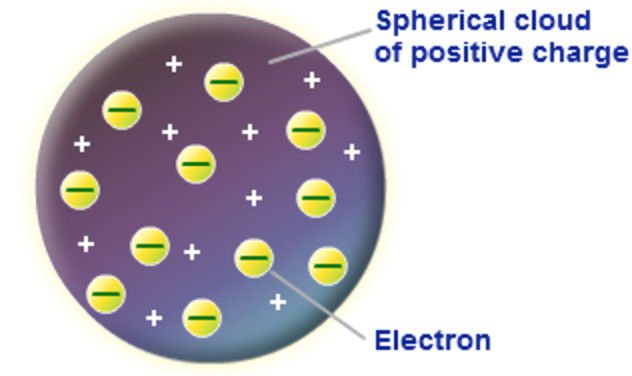
On discharge of electricity through gases, it became clear that an atom consists of positive and negative charges. J.J. Thomson tried to explain the arrangement of positive charge and the electrons inside the atom. Apple os x base system. According to him, an atom is a sphere of positive charge having a radius of the order of 10-10m. The positive charge is uniformly distributed over the entire sphere and the electrons are embedded in the sphere of positive charge as shown in Fig 6.6. The total positive charge inside the atom is equal to the total negative charge carried by the electrons, so that every atom is electrically neutral.
According to Thomson, if there is a single electron in the atom (like a hydrogen atom), the electron must be situated at the centre of the positive sphere. For an atom with two electrons (helium atom), the electrons should be situated symmetrically with respect to the centre of the sphere i.e., opposite sides of the centre at a distance of r/2 , where r is the radius of the positive sphere. In a three electron system of theatom, the electrons should be at the corners of a symmetrically placed equilateral triangle, the side of which was equal to the radius of the sphere. In general, the electrons of an atom are located in a symmetrical pattern with respect to the centre of the sphere.
It was suggested that spectral radiations are due to the simple harmonic motion of these electrons on both sides of their mean positions. Moreover, the stability of the atom was very well explained on the basis of this model.
Drawbacks

Thomson's Model Of The Atom
i.According to electromagnetic theory, the vibrating electron should radiate energy and the frequency of the emitted spectral line should be the same as the electron. In the case of hydrogen atom, Thomson's model gives only one spectral line of about 1300 Å. But the experimental observations reveal that hydrogen spectrum consists of five different series with several lines in each series.
Thomson Atomic Theory
ii.It could not account for the scattering of α-particles through large angles.
